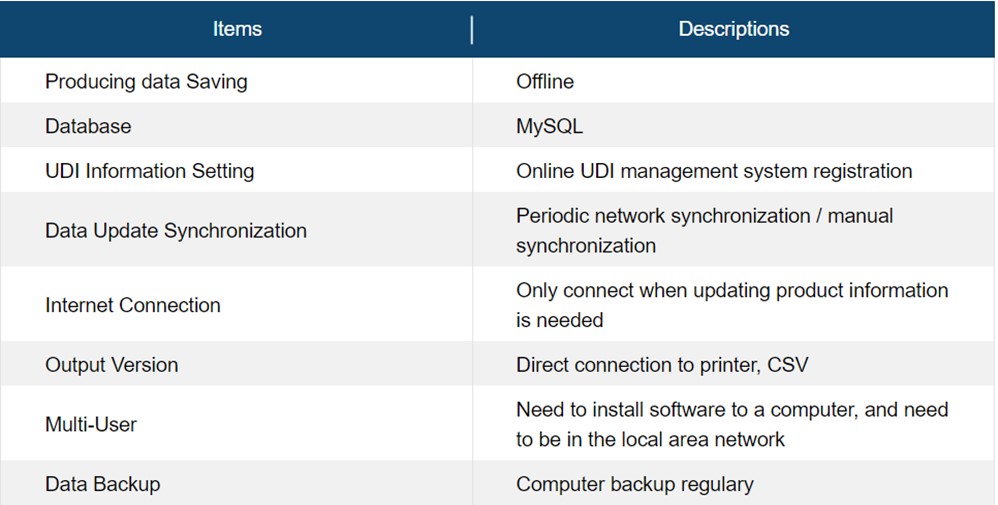
*Easy to use, UDI regulations of various countries
*Multiple company codes and multiple DI, which is convenient and efficiency
*The private database is convenient for recording and sorting, and the information won’t be exposed and leaked. Good job of label management.
*System error-proof design, eliminating human error or file deletion and tampering.
*The high integration for label machines and code scanners can print and detect on the production line directly.
*Software is automatically updated, and don’t miss the latest regulatory requirements and new features upgrade.
*Provide UDI work instructions, procedures, and IQ/OQ/PQ documents, in line with QMS and ISO13485.
*Simply inventory management function for simultaneously doing UDI management, label printing, and inventory management.
*Provide label editing without purchasing additional editing software
*Support multi-country UDI database format files, easy to upload